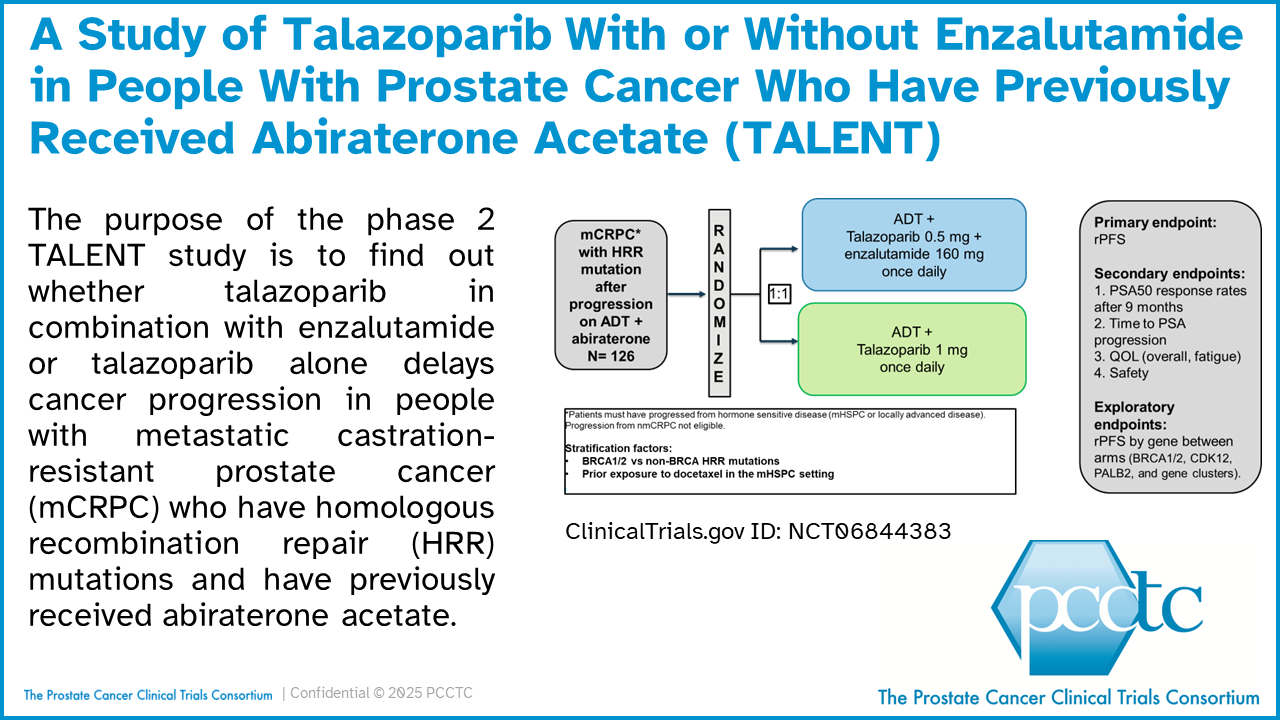
The PCCTC’s Study of Talazoparib With or Without Enzalutamide in People With Prostate Cancer Who Have Previously Received Abiraterone Acetate (TALENT) Activated
The PCCTC-managed Randomized Open-label Phase 2 Study of TALazoparib With or Without ENzaluTamide in Patients With Metastatic Castration-Resistant Prostate Cancer and HRR Mutations After Progression on Abiraterone Acetate (TALENT; ClinicalTrials.gov ID: NCT06844383) has been activated at Dana-Farber Cancer Center. The purpose of this TALENT is to find out whether talazoparib in combination with enzalutamide or talazoparib alone delays cancer progression in people with metastatic castration-resistant prostate cancer (mCRPC) who have homologous recombination repair (HRR) mutations and have previously received abiraterone acetate. TALENT will soon also open at Memorial Sloan Kettering Cancer Center.